Expertise Therapeutic Areas
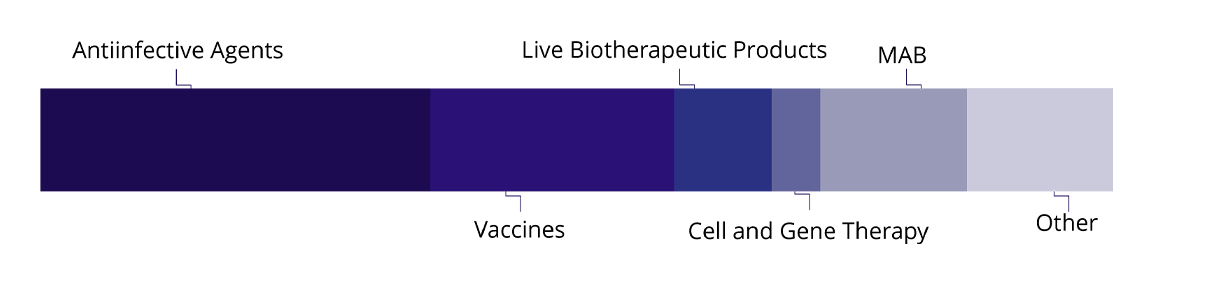
Infectology

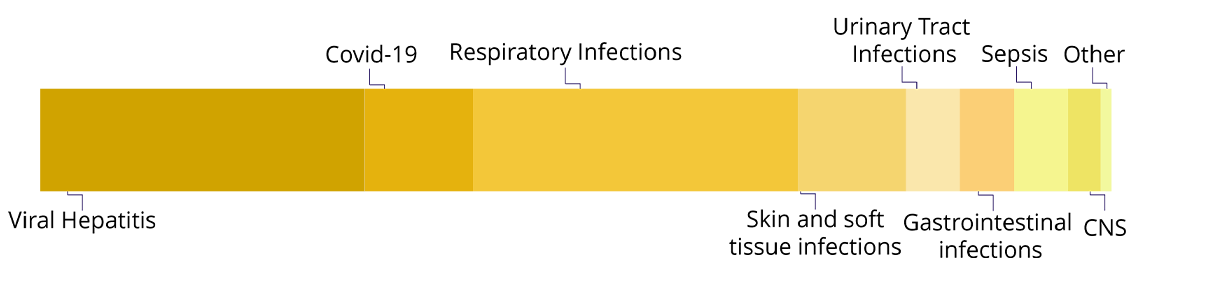
Infectious Diseases
Over 20 years of experience in various types
of infections including isolated and systemic conditions such as sepsis
Accelsiors’ team has supported development of several robust study designs for infectious disease studies, taking into account various factors such as the target population, seasonality of the disease, epidemiological considerations, vaccination status, sample size, control groups, endpoints, and statistical analysis plan. We believe that rigorous study design is essential for generating reliable and meaningful results.
Collaboration among multiple stakeholders is crucial in infectious disease studies, and we actively foster partnerships with researchers, healthcare providers, public health agencies, regulatory authorities, sponsors, and patient advocacy groups. Our global network of study sites and access to diverse patient populations enable us to capture a comprehensive understanding of disease manifestations, treatment responses, and potential adverse events.
Patient recruitment and retention are key challenges in infectious disease studies, and we employ effective strategies to ensure timely enrollment. In infectious disease studies, flexibility and adaptability are crucial due to rapidly evolving situations such as outbreaks or emerging pathogens. Our dedicated teams are prepared to modify study protocols, procedures, or recruitment strategies in response to emerging data or external factors to maintain the study’s relevance and effectiveness.
Accelsiors supports the development of robust infectious disease study designs, cultivating collaborations with key stakeholders, ensuring timely patient recruitment, and flexibly adapting to evolving situations to generate reliable and meaningful results, while maintaining study relevance and effectiveness.
During the COVID-19 pandemic, we have been actively involved in COVID-19 and long COVID clinical research, managing global phase II and phase III studies. We have in-depth knowledge of country-specific regulatory requirements and a vast network of investigators and sites. We closely monitor additional external factors related to COVID-19, such as population inoculation percentages and variants, to ensure study feasibility and relevance.
Accelsiors is committed to advancing infectious disease research and development, and we are ready to collaborate with you on your infectious disease studies. Our expertise, global network, and dedication to scientific excellence and patient safety make us a trusted partner in this important field.
Vaccines
We say with pride that we have vast experience in vaccine development and infectious diseases since our foundation in 2002, designing and conducting phase I to phase III studies in pediatric, adult, and elderly populations. Creating new vaccines is a slow, systematic, expensive, and laborious process that requires coordination between science, medicine, public health, vaccine developers, and society. Vaccines are, however, the most effective public health tool for controlling infectious diseases.
Accelsiors and its senior managers have been at the forefront of vaccine development for over 20 years. Vaccine development is still very actual, as there are many new and re-emerging pathogens for which we do not have effective vaccines. Vaccine development faces several challenges, for which Accelsiors is well prepared and can support our clients in any aspect of vaccine development. We have a deep understanding of not only conventional vaccines but also the regulatory implications and potential benefits and safety hazards of novel approaches such as vaccinomics, reverse vaccinology, and structure-based vaccine design. Accelsiors has been involved deeply in the development of different prophylactic vaccines. Our team has conducted numerous phase I, II, and III studies of vaccines targeting infectious diseases, such as seasonal influenza, rabies, and varicella zoster.
Boasting over two decades of experience, Accelsiors has been always engaged in vaccine development and infectious diseases, executing comprehensive phase I to III studies and tackling complex challenges in the vaccine field, with a profound understanding of both conventional and novel vaccine design approaches, and a proven track record in delivering successful outcomes for various prophylactic vaccines.
Live Biotherapeutic Products (LBP)
The realm of microbiome-based medicinal products is an exciting frontier in research and development, with potential benefits extending well beyond gut health to areas like metabolic and immune disorders, skin conditions, and even mental health.
As a trailblazer in Live Biotherapeutic Products (LBP) clinical research, Accelsiors not only actively contributes to this evolving field but also holds a significant role in the Pharmabiotic Research Institute (PRI), the EU Microbiome Regulatory Science Center. By assigning representatives to PRI’s board of directors, we actively influence and participate in shaping the future of microbiome research.
Accelsiors is a pioneering force in the thrilling frontier of microbiome-based medicinal products research, actively shaping the future of the field through key roles in the Pharmabiotic Research Institute and contributing to advancements in areas from gut health to mental well-being
Our approach to Infectious Diseases

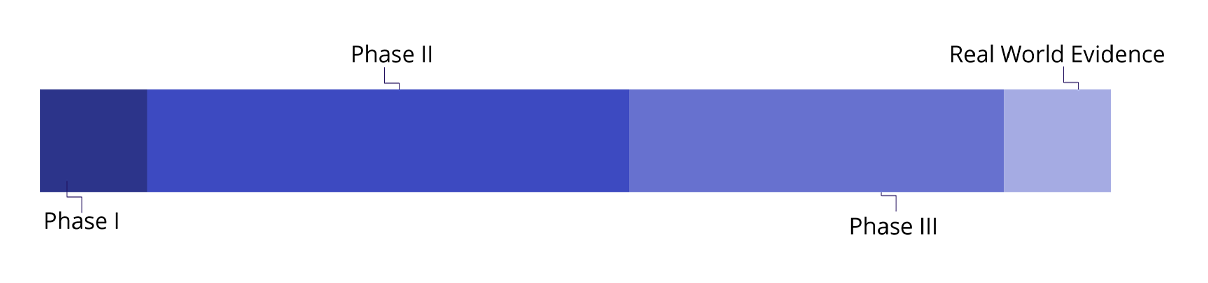
- Executed studies from phase I (first in human) to phase III (registration) trials
- Detailed database of highly qualified infectology sites worldwide
- Access to patients around the globe
- Significant experience in translational science in the field of infectology
- Depth of experience with population PK analysis in adult and pediatric population, including PK/PD modelling
We offer flexible cooperation models: