Expertise Multidisciplinary Areas
Cell and Gene Therapy

Join Accelsiors: Advancing the Frontiers of Cell and Gene Therapy Development
Integrating Knowledge, Innovation and Success
At Accelsiors, we take pride in leading the charge in Cell and Gene Therapy (CGT) development. Our exceptional team seamlessly amalgamates knowledge, innovation, and a proven track record of success, driving your CGT program to new heights.
Innovation and Expertise: A Matchless Convergence
Accelsiors stands out as a trusted partner for biotech companies, thanks to our unwavering commitment to excellence, rigorous scientific approach, and operational efficiency. We’re driven by a shared goal: improving patient outcomes through the development of groundbreaking therapies.
Staying Ahead in a Rapidly Evolving Field
We comprehend the crucial need to remain on the cutting edge of the fast-evolving field of cell and gene therapies. As these therapies revolutionize disease treatment and potential cures, they promise improved healthcare outcomes. With over 50 ongoing cell/gene therapy development programs, the demand for expertise in this area has never been greater.
Your Dedicated CGT Team
At Accelsiors, we assign a dedicated CGT team to support your clinical study. This specialized team provides a laser-focused approach at each stage of product development, infusing our collective knowledge and insights into your product’s success.
Drawing on Diverse Expertise
Our team encompasses experts from translation science, medicine, regulatory affairs, CMC (chemistry, manufacturing, and controls), biostatistics, clinical pharmacology, biomarker science, operational delivery, assay validation, quality requirements, and AI and data sciences. This diverse expertise allows us to provide comprehensive support tailored to the unique challenges of CGT development.
Excellence at Every Step of the CGT Journey
Our commitment to excellence permeates every aspect of the CGT journey. With an in-depth understanding of the regulatory landscape surrounding cell and gene therapies, we can navigate the intricate requirements to ensure successful product development. Our expertise in biostatistics, clinical pharmacology, and biomarker science ensures robust study designs and meaningful measurement of therapeutic responses. Additionally, our operational delivery capabilities, coupled with the application of AI and data sciences, ensure smooth execution of clinical trials and valuable insights.
Accelsiors: Your Trusted Partner in CGT
In partnering with Accelsiors, you gain the combined advantage of cutting-edge innovation and unparalleled expertise in cell and gene therapy development. Together, we can push the boundaries of medicine, bring transformative therapies to patients, accelerate the development of next-generation therapies, and leave a lasting impact on the biotech industry and healthcare.
Our steadfast commitment to excellence, scientific rigor, and operational efficiency distinguishes Accelsiors as a trusted partner for biotech companies.
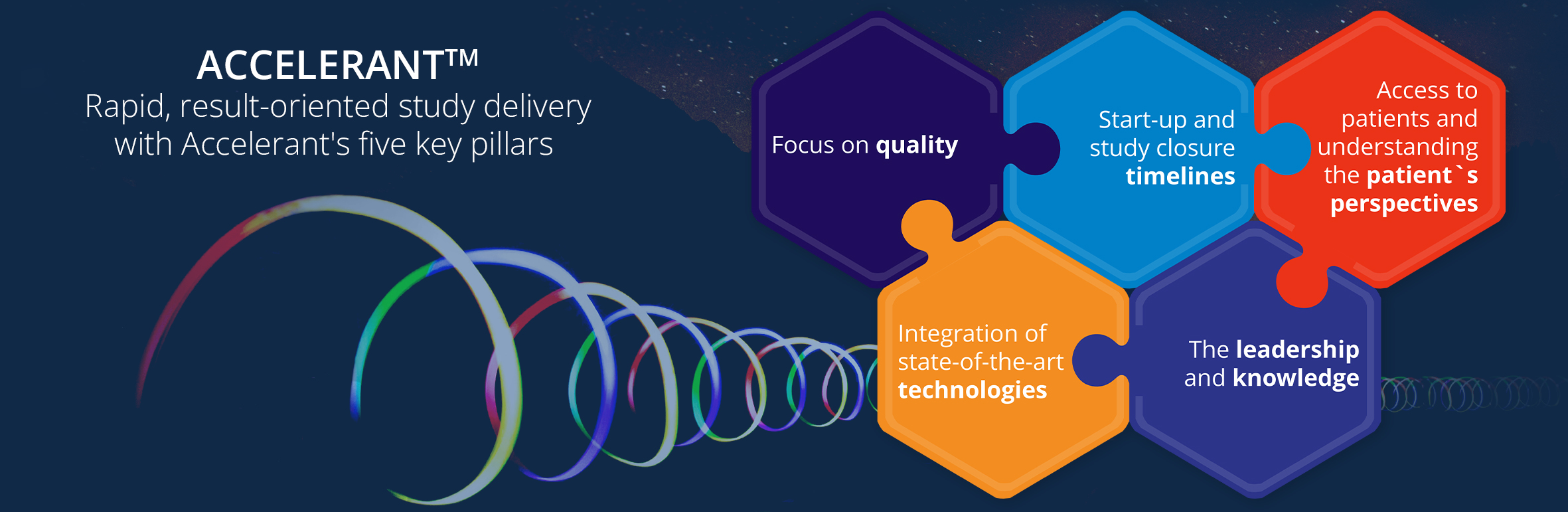
The successful execution of CGT clinical research requires careful planning, efficient implementation, and effective management. We will ensure at Accelsiors:
- A robust study design to clearly articulate the research question and generate reliable results,
- Maximal regulatory compliance and compliance with ethical and GCP principles to protect patient safety, ethical integrity, and data quality,
- A precise site and investigator selection to facilitate smooth study conduct, patient recruitment, and data collection,
- A reliable patient recruitment and retention strategy to preserve the study conduct timelines and ensure the reliability of its outcome,
- A highly efficient safety monitoring and prompt Adverse Event reporting to ensure participant safety, enabling timely intervention or modification of the study protocol, if necessary,
- Superb data quality to ensure data accuracy and completeness,
- Competent project management and study oversight to coordinate study activities, manage timelines, and ensure adherence to the study protocol and regulatory requirements,
- Competent Vendor selection to ensure the safe delivery of the study medication and accurate and reproducible laboratory results provision
We offer flexible cooperation models:


