Expertise Multidiscipinary Areas
Biosimilars

Join Hands with the Trailblazers in Biosimilar Development!
Biosimilars: Affordable, Safe, and Effective
Developing and securing approval for biosimilars is key to offering patients a wider range of affordable, safe, and effective treatment choices. As a front-runner in this field, biosimilar drug development is a primary specialty of Accelsiors.
Unraveling the Complexity of Biosimilar Development
Our in-depth expertise and experience equip us to accelerate and expertly navigate the intricacies of any biosimilar development program. We acknowledge the need for efficiency to minimize study risks, curtail development costs, and shorten time to market.
Biosimilar Success Factors: Our Strategic Approach
Our strategy includes the identification of several critical success factors pivotal to Biosimilar programs. Our paramount objective is to aid our customers in selecting the most advantageous solution for developing their Biosimilars or superior biologics, known as “Biobetter.”
The Power of PD Biomarkers in Accelerating Biosimilar Development
Accelsiors is currently immersed in evaluating the potential of utilizing Pharmacodynamic (PD) biomarkers to expedite biosimilar development. PD biomarkers hold the potential to enhance the efficiency of biosimilar development programs, as the current procedure involving prolonged and costly efficacy clinical studies can be both financially and temporally taxing.
Incorporating PD Biomarkers: The Future of Biosimilar Approvals
Though PD biomarkers have not been widely employed in biosimilar approvals, there is considerable opportunity to integrate this approach as a replacement for or in combination with comparative clinical studies measuring efficacy endpoints in the future. PD biomarkers have been effectively used as primary endpoints in other drug development programs, serving either as a direct measure of clinical outcomes or a surrogate endpoint.
Collaborate with Innovators in Biosimilar Development!
Unique Expertise in Biosimilar and Biobetter Development
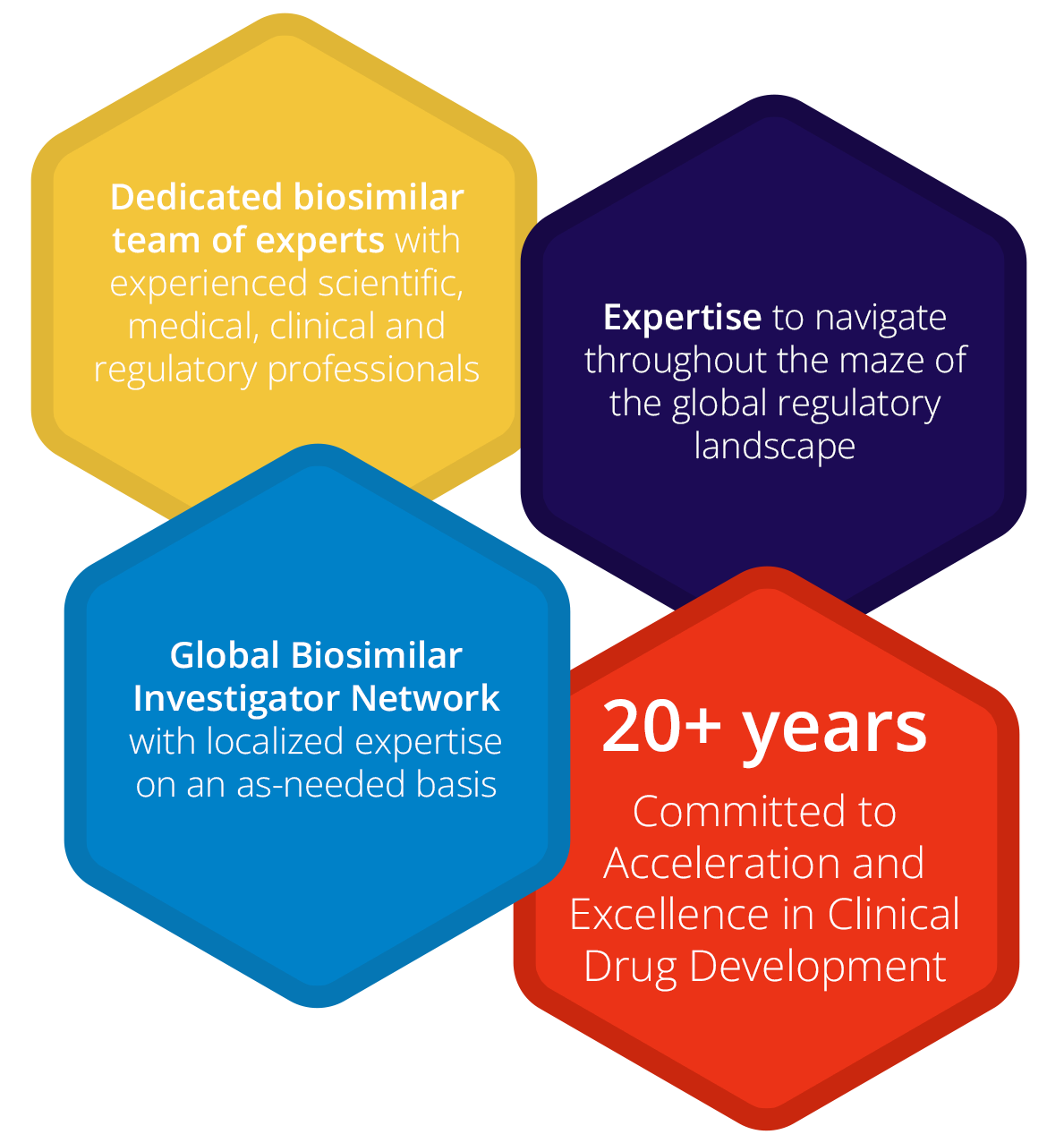
Accelsiors’ extensive experience in the development and registration of Biosimilars and Biobetters positions us among the few firms that understand the unique challenges, regulations, and opportunities in this arena.
Navigating the Biosimilar Landscape since 1996
Many of our professionals have been engaged in biosimilar drug development from the dawn of this innovative field in 1996, steering the world’s first biosimilar drug development projects. They provide support for clinical trials as well as product registrations in this domain.
Excellence in Biosimilar Drug Development
We comprehend the challenges, opportunities, and success factors of biosimilar drug development. We have the depth of experience, expertise, and the right capabilities to adeptly manage the complexity of your biosimilar study.
Reducing Time and Cost through Regulatory Expertise
Our regulatory connoisseurs can aid you in decreasing the time and cost of maneuvering through the intricate global regulatory landscape.
Accelerated Start-up and Patient Access
Our well-established relationships with sites and investigators experienced in biosimilars ensure your study’s rapid start-up and patient access.
Biosimilar Knowledge Network: Tap into Expert Opinions
Benefit from our special relationships with key opinion leaders in the biosimilar area.
Biosimilar Trial Designs Across Therapeutic Indications
Accelsiors’ experts have created numerous biosimilar trial designs and managed clinical studies across various therapeutic indications.
Our Track Record: Showcasing Depth and Breadth
We have conducted over 30 biosimilar studies, from first-in-human Phase I clinical trials to complex Phase III registration studies. Our track record spans ten biosimilars and several “bio better” development programs, from simple peptides to complex monoclonal antibodies, including the first modern long-acting rhGH. We have achieved multiple successful out-licensing and marketing authorizations of biosimilar products both in Europe, North America, and other regions around the world.

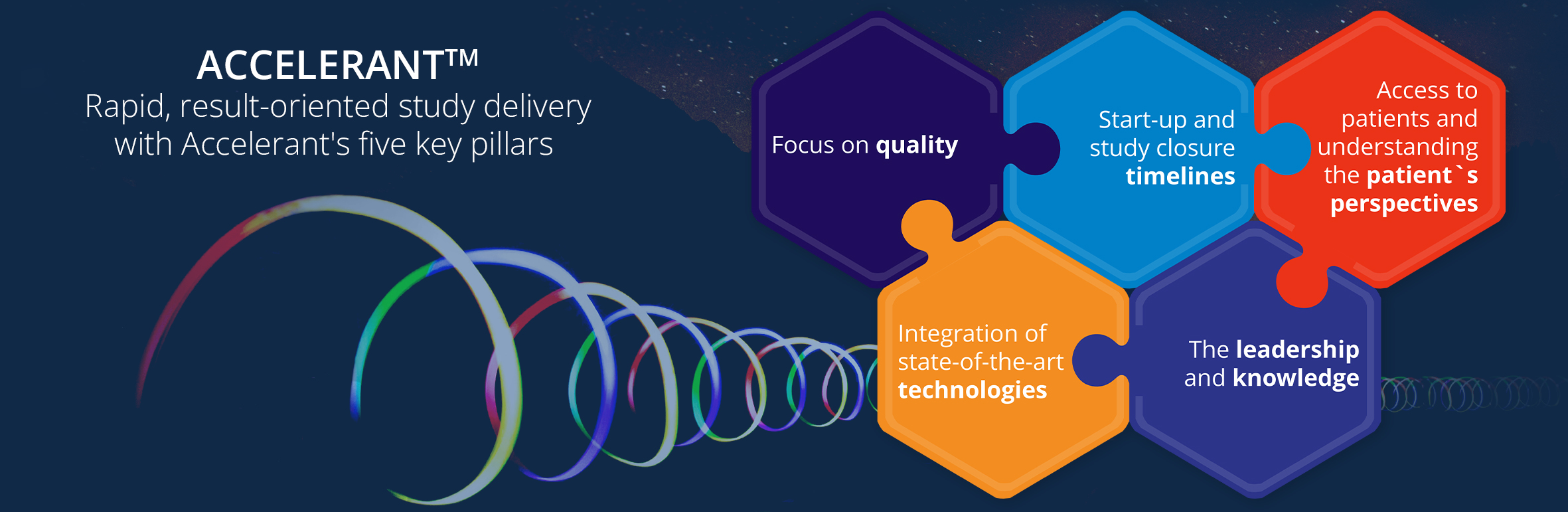
Accelsiors’ experts have built numerous biosimilar trial designs and managed clinical studies in various therapeutic indications.
We have conducted the following:
- over 30 biosimilar studies, from first-in-human Phase I clinical trials to complex Phase III registration studies
- Ten biosimilars and several “bio better” development programs from simple peptides to complex monoclonal antibodies, including the first modern long-acting rhGH
- Several successful out-licensing and marketing authorizations of biosimilar products both in Europe, North America, and in other regions around the world
We offer flexible cooperation models: