Expertise Multidiscipinary Areas
Orphan Disorders


Rare diseases affect more than 300 million lives around the globe, and nearly 7,000 disorders are described in this category. Two-thirds of these disorders are genetic disorders affecting the pediatric population with grave consequences, either life-threatening or resulting in severe disabilities.
Conducting clinical trials in orphan disorders is a great challenge. The patient population is dispersed across a sizeable geographical area, eligibility criteria are often severely restricted, and assessments must be patient friendly. In addition, there is a need for a more proper understanding of the disease’s natural history, selecting clinically meaningful endpoints, and in the lack of sensitive, non-invasive biomarkers and clinical outcome measures to have meaningful methods to monitor treatment responses in a particular disease.
With over 20 years of experience in the field, Accelsiors is well-positioned to support the planning and execution of clinical trials for rare diseases. We understand the unique challenges associated with these trials and have developed strategies to overcome them effectively.
Our global expertise, extensive networks, and available resources enable us to navigate the complexities of rare disease research and accelerate the development of treatments
At Accelsiors, we have dedicated ourselves to addressing the specific needs of rare disease trials. We have built our organization with the expertise and capabilities necessary to succeed in these challenging indications. Our team is equipped with the knowledge and experience to design robust study protocols, identify appropriate patient populations, and engage with key stakeholders in the rare disease community.
We recognize the urgency of finding effective treatments for individuals living with rare diseases. By leveraging our global expertise, networks, and resources, we can contribute significantly to the success of rare disease trials. Our goal is to expedite research efforts, facilitate collaboration among researchers, and ultimately improve outcomes for patients with rare diseases.
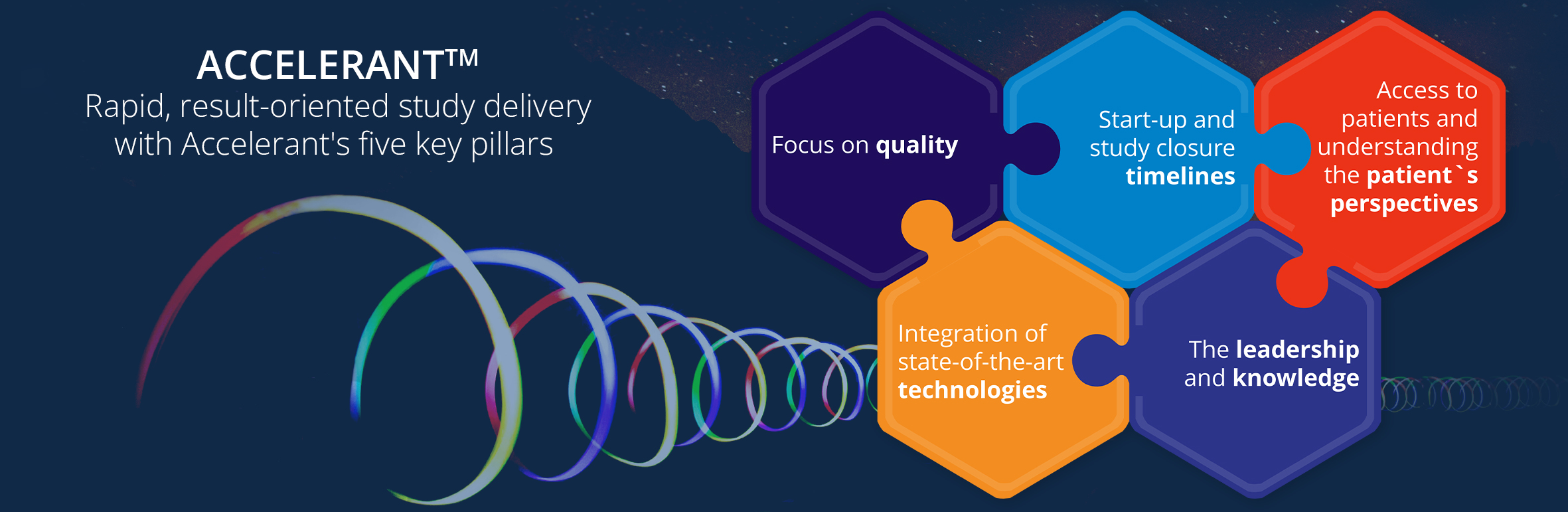
We have proudly introduced the Accelerant Program™ to improve the successful outcome of our Orphan disease clinical trials. Now, we have expanded this approach to most of our clinical research studies irrespective of indications. With the Accelerant Program™ we are embedding knowledge and compliance, quality, patients’ perspectives, speed, agility, state-of-the-art technologies, and cost-effectiveness in our clinical trials.
Accelsiors is committed in making the difference in the field of rare disease research. Partnering with us means accessing our extensive experience and dedicated approach, ultimately bringing us closer to developing life-changing therapies for individuals affected by rare diseases.
Several constraints are challenging the rare disease clinical trials’ planning and conduct. It requires deep expertise to find the proper trial design augmented with appropriately selected endpoints and sufficiently powered sample size, control group(s) selection, the most sensitive biomarkers, and the most comfortable trial assessments acceptable to patients involved, all this considering within highly proper ethical frames and financial constraints.
By leveraging our 20+ years of global expertise, networks, and resources, Accelsiors can significantly contribute to the success of rare disease trial planning and conduct. We can help overcome the unique challenges associated with these trials, accelerate research efforts, and ultimately facilitate the development of effective treatments for individuals living with rare diseases.
Accelsiors brings more than 20 years’ experience in planning and conducting rare clinical trials on a global scale. We built our organization to succeed in these challenging indications.
We have proudly introduced the Accelerant Program™ to improve the successful outcome of our Orphan disease clinical trials. Lately, we expanded this approach to most of our clinical research studies irrespective of indications. With the Accelerant Program™ we are embedding knowledge and compliance, quality, patients’ perspectives, speed, agility, state-of-the-art technologies, and cost-effectiveness in our clinical trials.
We offer flexible cooperation models:





